An ultrafiltration-collection probe to remotely and continuously monitor blood glucose and lactate in broiler chickens
B. Savenije1,2, E. Lambooij1, M.A. Gerritzen1 , K. Venema2 and J. Korf2
1
Department of Food Science, Institute for Animal Science and Health (ID-Lelystad),
Lelystad, The Netherlands
2 Department of Biological Psychiatry, University of Groningen,
Groningen, The Netherlands
To closely monitor physiological processes in blood or other body fluids it is important to frequently take samples for off line analysis. Such sampling techniques are often physically or psychologically stressful, or pose a severe restraint on the circumstances under which sampling can be done. Animals often need to be restrained to take blood or tissue samples. For small animals repeated sampling can imply a compromise of their physiological capacity to compensate for the loss. Even if samples can be taken stress-free, animals must often be housed individually in an experimental environment that allows easy sampling.
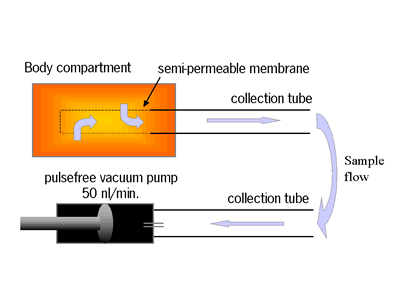
Recently we have developed an ultrafiltration-collection probe for the sampling of blood or (subcutaneous) tissue fluid that may be used for continuous, stress-free sampling in a naturalistic environment. The system is based on inserting into the relevant body compartment a semipermeable membrane, which is connected to a collection tubing that stores the fluid sample. Its small inner diameter (125 mm) prevents diffusion. A low, continuous sampling flow (50 nl/min.) is driven by a pulse-free vacuum pump (Figure 1) [2]. As only tissue fluid is stored, the ultrafiltrate always has 100% recovery. The membrane (cut-off point 20 kD) keeps out degrading components. The entire system is small and may be attached to the animal. Afterwards the ultrafiltration-collection probe is removed and the contents are analyzed in 20 nl fractions using a bi-enzyme reactor (glucose oxidase/horseradish peroxidase or lactate oxidase/horseradish peroxidase, using a ferrocene buffer as mediator) with electrochemical detection in a flow injection system [1], resulting high resolution glucose and lactate profiles.

Figure 1. Schematic diagram of the ultrafiltration-collection probe and sample flow.
Recently this technique was applied to sample blood ultrafiltrate for 8 hours in 40 freely moving, group housed chickens to monitor glucose and lactate levels. A membrane was surgically inserted into the right wing vein. The collection tubing and pump were attached under the wing in a plastic bag. After recovery the birds were returned to their pens. The probe did not seem to hinder the chickens, nor attract unwanted attention from other chickens. At 3 days after surgery the pump was started, and the probe ran for 8 hours during which the birds were crated and transported for 1,5 hours. Afterwards the probe was removed and the collection tubing separated, sealed and stored at 4 °C until analysis. The sample was analyzed to give a 5-minute resolution profile of glucose and lactate (Figure 2).
Profiles of blood components are well suited for combination with behavioral observations. The high time resolution may provide data on fast transient effects, like increased glucose mobilisation during social conflicts, when combined with time-dependent behavioral observations of animal interactions. Circadian rhythmicity might give insight in the effects of with day/night activity or feed intake. The ultrafiltration-collection probe is an aspecific sampling system. In the future it may be further developed for sampling in different tissues, or different components, e.g. catecholamines, which will allow the study of behavioral and physiological stress responses in numerous experimental and naturalistical settings.
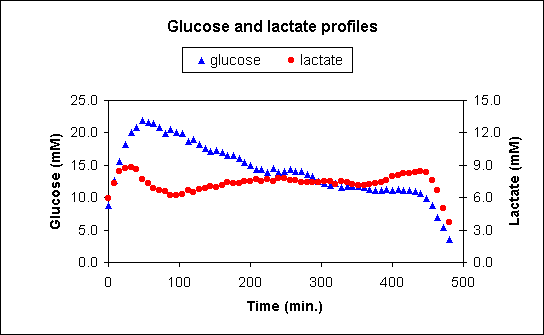
Figure 2. An example 8-hour profile of glucose and lactate levels (in mM) in the ultrafiltrate, as measured in a group-housed chicken. Each point represents 1 measurement per 8 minutes sampling time (mean flow = 31.25 nl/min).
Paper presented at Measuring Behavior 2000, 3rd International Conference on Methods and Techniques in Behavioral Research, 15-18 August 2000, Nijmegen, The Netherlands
© 2000 Noldus Information Technology b.v.